Name the type of bond shown and explain how it is formed?
2 Answers
These aren't bonds. These are intermolecular interactions called hydrogen "bonds", which are, on the whole, significantly weaker than a typical chemical bond.
They are, in fact, in BETWEEN molecules, NOT within molecules.
A hydrogen-"bonding" interaction forms when a particularly electronegative atom polarizes electron density away from a particularly electropositive
We tend to describe the following interactions as hydrogen-"bonding"...
#-stackrel(delta^(-))(ddot"N")-stackrel(delta^(+))"H" stackrel("+"->)(cdotcdotcdot) :stackrel(delta^(-))"N"-#
#-stackrel(delta^(-))(ddot"O")-stackrel(delta^(+))"H" stackrel("+"->)(cdotcdotcdot) :stackrel(delta^(-))"O"-#
#-stackrel(delta^(-))(ddot"F")-stackrel(delta^(+))"H" stackrel("+"->)(cdotcdotcdot) :stackrel(delta^(-))"F"-#
...where the arrow,
Here, we call the
There are other more obscure examples, however, so hydrogen-"bonding" is not limited to the above three common cases.
Here is one case of a weak interaction:
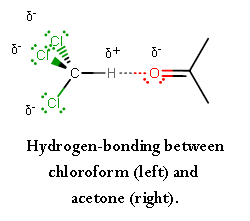
The three chlorine atoms are electron-withdrawing groups, which make the hydrogen very electropositive.
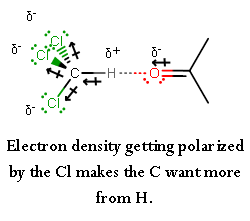
This is enough for the carbonyl oxygen on acetone to become a hydrogen-"bond" acceptor, polarizing the hydrogen atom and forming a hydrogen-"bonding" interaction.
The diagram represents
Explanation:
Hydrogen bonding is an INTERMOLECULAR interaction, i.e. it occurs BETWEEN molecules when hydrogen is covalently bound to a strongly electronegative element, such as oxygen, or nitrogen, or fluorine. Clearly the blue-hatched bonds in the diagram are intermolecular.....
When hydrogen is covalently bound in an isolated molecule, charge separation occurs so that a dipole occurs......for water we could represent this as......
.........when the dipoles line up in solution.....
In each case, the electronegative oxygen atom binds to electropositive hydrogen in an adjacent molecule the which has been polarized by the oxygen in the same molecule.....
This acts as a potent intermolecular force of attraction and thus it tends to elevate boiling points. For its size, the normal boiling point of water is ABSURDLY HIGH at
Hydrogen bonding is so strongly expressed in the first members of each group, because these,

If you can't see the caption the alien is desperate for a cooling drop of ammonia.......


