Please let know how is Electronegativity value in Halogen family? I am really unclear. Thank you.
2 Answers
The electronegativity values for the VIIA group or Halogen value are the highest in their period or row, and decrease as the atomic number of the Halogen increases.
Explanation:
The group VIIA or Halogens have a valance electron configuration of
This gives the Halogens 7 valance electrons. One more electron will give the Halogens the same electron configuration of the VIIIA group also called the noble or inert gases. Gaining that one more electron will cause the Halogen to achieve a great level of stability.
The electron configuration causes the Halogens to have a strong pull for one more electron. The definition of electronegativity is the pull of the element for more electrons. Thus the VIIA or Halogens will have the highest electronegativity of any element in their row, or period.
Florine number 9
The electronegativity of the Halogens decreases as the elements get larger and therefore less reactive.
Here are the electronegativities of the halogens.
Explanation:
Below is a diagram of electronegativity trends in the Periodic Table.
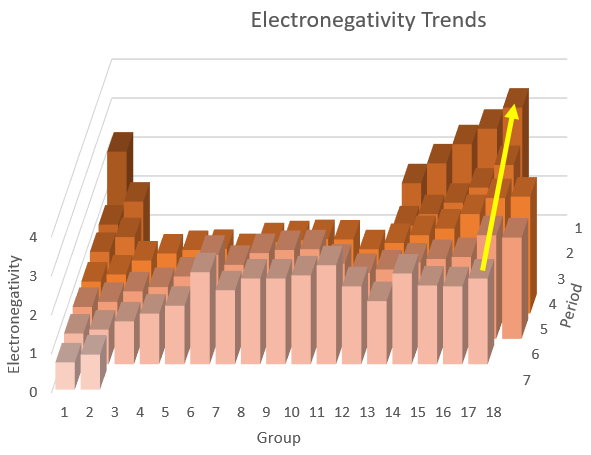 Electronegativity trends
Electronegativity trends
The trends for the halogens (in Group 17) are shown by the yellow arrow.
The electronegativities increase from the bottom to the top of the Group, and
Electronegativity tends to increase from left to right in a Row of the Periodic Table.
With the exception of astatine (in Row 6), each halogen has the highest electronegativity of any element in its Row.

