The atom cobalt has 27 electrons. How many energy levels will its electrons use?
1 Answer
Four energy levels.
Explanation:
The number of electrons each energy level can hold increases as you add more and more energy levels to an atom.
The relationship that exists between the energy level,
color(blue)(|bar(ul(color(white)(a/a)"no. of e"^(-) = 2n^2color(white)(a/a)|)))
You can use this equation to find the maximum number of electrons that can be added to each energy level. You will have
- the first energy level,
n=1
"no. of e"^(-) = 2 * 1^2 = "2 e"^(-)
- the second energy level
n=2
"no. of e"^(-) = 2 * 2^2 = "8 e"^(-)
- the third energy level,
n=3
"no. of e"^(-) = 2 * 3^2 = "18 e"^(-)
- the fourth energy level,
n=4
"no. of e"^(-1) = 2 * 4^2 = "32 e"^(-)
and so on.
In your case, cobalt,
Now, it's very important to remember that when you're adding electrons to an atom, the 3d-orbitals, which are located on the third energy level, are higher in energy than the 4s-orbital.
This means that you must fill the 4s-orbital first, then distribute the rest of the electrons to the 3d-orbitals.
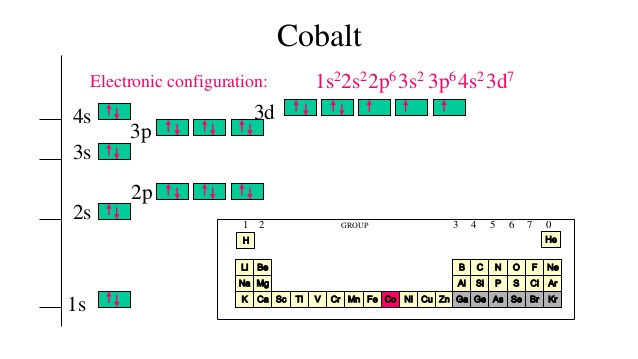
So, a neutral cobalt atom will have
n=1 -> "2 e"^(-) in the1s subshell
n=2 -> "8 e"^(-) in the2s and2p subshells
Now, these two energy levels will hold
"2 e"^(-) + "8 e"^(-) = "10 e"^(-)
Now comes the tricky part. The third energy level can hold
"27 e"^(-) - "10 e"^(-) = "17 e"^(-)
that the neutral cobalt atom has. You could thus say that
color(red)(cancel(color(black)(n=3 -> "17 e"^(-)))) in the3s ,3p , and3d subshells
and conclude that the electrons that surround the nucleus of a cobalt atom are spread out on
Taking it one subshell at a time, you will have
"2 e"^(-) -> in the3s subshell
"6 e"^(-) -> in the3p subshell
You now have
"17 e"^(-) - ("2 e"^(-) + "6 e"^(-)) = "9 e"^(-)
to distribute. Because the 4s orbital is filled before the 3d-orbitals, the next two electrons are going to be distributed on the fourth energy level
n=4 -> "2 e"^(-) in the4s subshell
The remaining
Therefore, a neutral cobalt atom will have
n=1 -> "2 e"^(-) in the1s subshell
n=2 -> "8 e"^(-) in the2s and2p subshells
n= 3 -> "15 e"^(-) in the3s ,3p , and3d subshells
n=4 -> "2 e"^(-) in the4s subshell