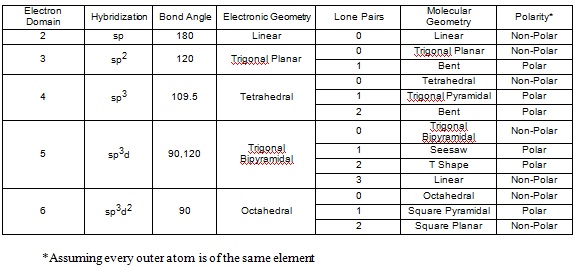
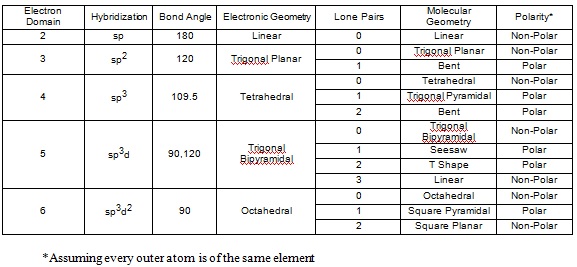
The charge clouds both methane, #CH_4#, and ammonia, #NH_3#, are arranged in a tetrahedral geometry. Why are the bond angles in methane 109.5 degrees while the bond angles in ammonia are 107.3 degrees?
1 Answer
Mar 2, 2017
Lone Pairs
Explanation:
In methane, the carbon has 4 valence electrons. It shares it with the one valance electron hydrogen has.
This means it has 4 bonds.
In ammonia, nitrogen has 5 valence electrons and shares 3 of them with the 3 hydrogens.
This means that it has 3 bonds and 1 lone pair.
A general note is that lone pairs occupy more space that regular bonds, which is why the bond angle for ammonia is a bit smaller than that of methane's.
PS. Methane is tetrahedral, while ammonia is trigonal pyramidal if you want to get more specific.
( )
)

