The combustion of propane...?
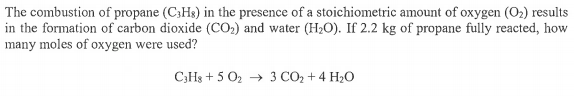
1 Answer
Jul 5, 2018
You have the stoichiometry...
Explanation:
And clearly, by the stoichiometry if
i.e. a mass of
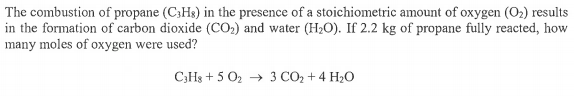
You have the stoichiometry...
And clearly, by the stoichiometry if
i.e. a mass of