Use valence bond theory to write the hybridization and bonding scheme for NCCH3. Sketch the model with the right geometry?
1 Answer
Warning! Long Answer. Here's what I get.
Explanation:
Step 1. Draw the Lewis structure
(a) Start with a skeleton structure.
The two
 Skeleton
Skeleton
(b) Attach the hydrogen atoms.
The question gives you a clue where they go.
The formula
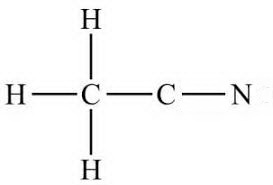 Connectivity
Connectivity
(c) Add electrons so that every atom gets an octet.
There is only one good way to do it: put a triple bond between the
 archives.library.illinois.edu
archives.library.illinois.edu
Step 2. Use VSEPR theory to predict the geometry about each atom.
- The terminal
C atom: four electron domains (3C-H bonds and aC-C bond).
∴ Tetrahedral. - The central
C atom: two electron domains (aC-C bond and aC≡N bond).
∴ Linear. - The
N atom: two electron domains (aC≡N bond and a lone pair)
Step 3. Assign hybridizations to each atom
- The terminal
C atom: tetrahedral. ∴sp3 hybridized. - The central
C atom: linear. ∴sp hybridized. - The
N atom: two electron domains. ∴sp hybridized.
Step 4. Sketch the orbitals involved
Here is my drawing (apologies! I'm not a graphics artist).
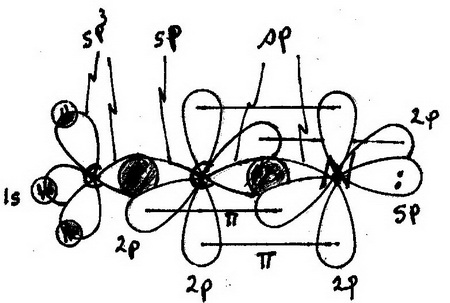 Orbitals
Orbitals
My drawing may be confusing, so here is a description:
H atoms: unhybridized1s orbitals- Terminal
C atom:sp3 hybridized; bond angles 109.5° C-H bonds: σ bonds formed by overlap ofH 1s andC sp3 orbitals- Central
C atom:sp hybridized C-C bond: σ bond formed by overlap ofC sp3 andC sp orbitalsN atom:sp hybridized.C≡N bond: σ bond formed by overlap ofC sp andN sp orbitals, plus two bonds formed by side-on overlap of unhybridized2p orbitalsC-C-N bond angle = 180°
Here's a model of the molecule for comparison:
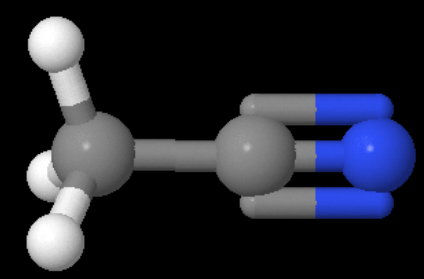 Model
Model

