What are all of the ions that may be formed when #H_3PO_4# ionizes in water?
1 Answer
Explanation:
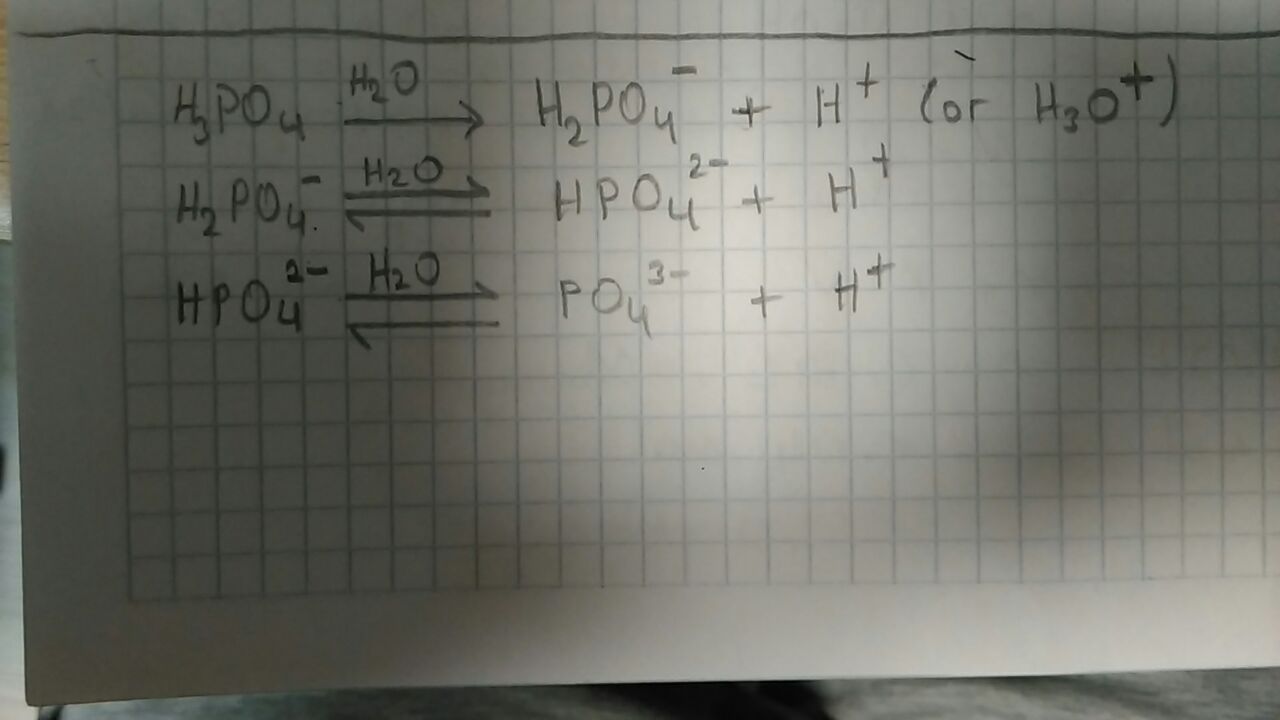
In first reaction one way arrow is used.
Because phosphoric acid is strong acid, it dissolves totally. Thus all amount of reactants consumed to produce all amount of products. So that, reaction always goes products side. No back way reaction. Therefore, only one way arrow, which goes to products side, is used.
Because


