What are dipoles? how are they related to van der waals forces?
i don't understand what the dipoles are and how they relate to the vanderwaals forces.
Please help me!
i don't understand what the dipoles are and how they relate to the vanderwaals forces.
Please help me!
1 Answer
Explanation:
A dipole forms when electric charge is separated, i.e. polarized. The modern covalent bond is considered to be a region of high electron density BETWEEN two positively charged nuclei
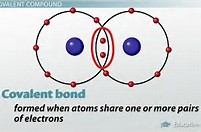
The covalent bond is thus conceived to be the sharing of two electrons: the bond is a region of high electron density that negates the electrostatic repulsion between 2 positively-charged atomic nuclei, such that a net attractive force results.
Because homonuclear diatomic molecules, i.e.
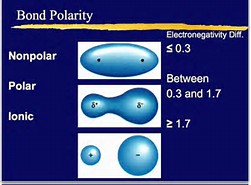
The illustration tries to represent non-polar, versus polar covalent, versus ionic interaction (where the electron transfers). (And their cut-off points are reasonably arbitrary). The important points to realize that where 2 atoms are covalently bound, if one atom is more electronegative than the other, charge separation occurs, i.e. to give
As an undergraduate you have to know that molecular polarity is addressed as the vector sum of the individual bond dipoles:
Hydrogen bonding could be considered another example of dipole-dipole interaction, and certainly such interactions is conceived to elevate the boiling points of

