What are the different types of optical isomers seen in coordination compounds? How do you draw optical isomers of coordination compounds?
1 Answer
Here's what I get.
Explanation:
Monodentate ligands
Complexes with monodentate ligands are classified as C (clockwise) or A (anticlockwise).
An example is the hypothetical complex ion
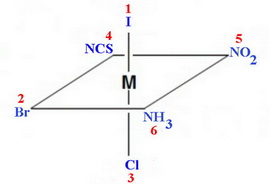
(From Department of Chemistry, UWI, Mona)
You assign priorities to the ligands per the usual Cahn-Ingold-Prelog rules.
Arrange the complex so the highest-priority ligand is at the top.
Viewing from the top, you look at the ligands in the horizontal plane.
You designate the isomers as C or A according to whether the direction from the highest to the next-highest priority ligand in the plane is clockwise or anticlockwise.
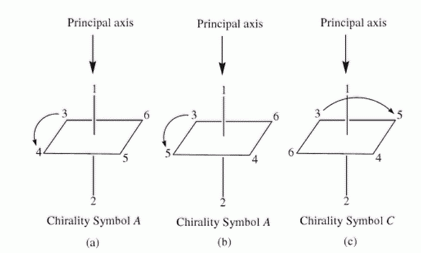
(From Department of Chemistry, UWI, Mona)
Our hypothetical complex was the C isomer.
Δ and Λ isomers
Optically active bis- and tris-bidentate complexes are said to have a screw chirality.

(From CSB | SJU Employees Personal Web Sites - College of Saint Benedict)
You arrange them so they look like left- or right-handed screws.
If you must rotate them clockwise to screw them into the paper, they are classified as Delta Δ (right-handed).
If you must rotate them counterclockwise, they are Lambda Λ (left-handed).
An example is the trisoxalatoferrate(III) ion.

You must twist the left-hand image to the left to screw it into the paper, so it is the Λ isomer.
The other image is like a right-hand screw, so it is the Δ isomer.

