What are the electron geometry and the molecular geometry of water?
1 Answer
The electronic geometry gives water a tetrahedral shape.
The molecular geometry gives water a bent shape.
Explanation:
Electronic geometry takes into account the electron pairs that are not participating in bonding, and the electron cloud density.
Here the 2 bonds of hydrogen count as 2 electron clouds, and the 2 electron pairs count as another 2, giving us a total of 4. With 4 electron regions, the VSEPR electronic geometry is tetrahedral.
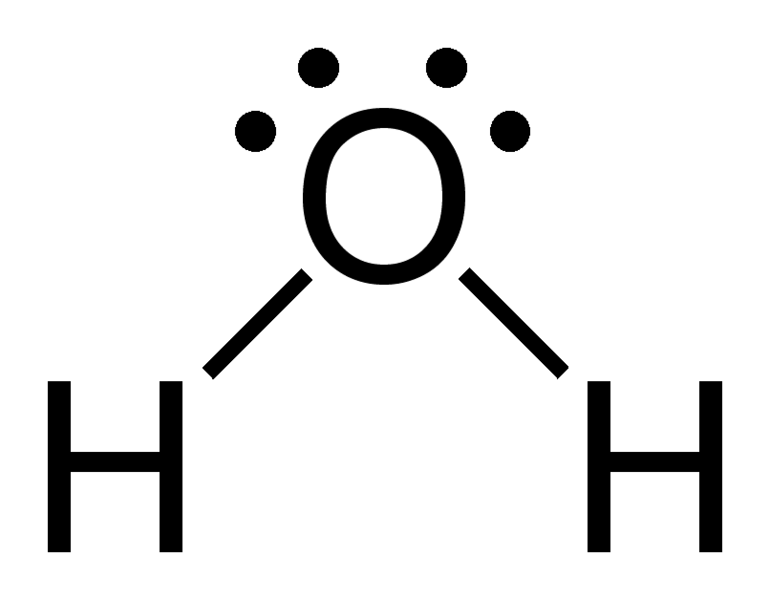
Molecular geometry looks at only those electrons that are participating in bonding. So here, only the 2 bonds to H are taken into account.
The shape would not be linear, as in the case with
There is electron repulsion going on so electrons take on shapes that help to reduce electron repulsion.


