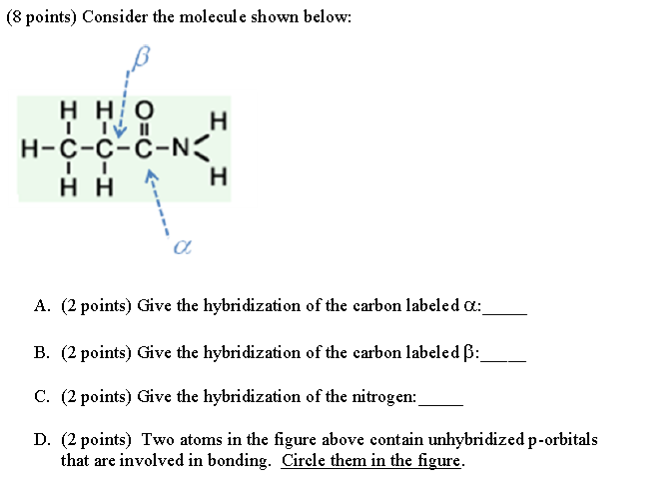
What are the hybridizations here?
1 Answer
Dec 3, 2016
A.
Explanation:
A.
The α carbon has no lone pairs and is directly attached to three atoms.
Its hybridization is
B.
The β carbon is directly attached to four atoms.
It is
C.
The nitrogen atom is

D.
Three atoms contain unhybridized
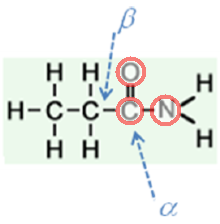
All three are involved in resonance of the amide group; all three are

