What are the two main types of chemical bonds?
1 Answer
Just to retire this question...these are (i)
Explanation:
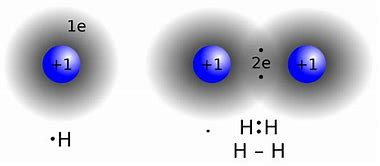
The modern covalent bond is conceived to be a region of high electron density between 2 positively charged atomic nuclei, such that internuclear repulsion is minimized, and a net attractive force results....and we could map this electron density pictorially..
 sites.google.com
sites.google.com
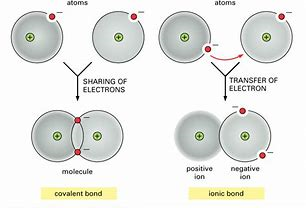
...clearly, there is maximum electron density BETWEEN the nuclei, cementing the bond...
 socratic.org
socratic.org
Ionic bonds fall outside this umbrella..and are conceived to result from the TRANSFER of electrons between atoms to give discrete positive and negative ions. And thus there is electrostatic interaction between particles... Given this bonding conditions, ionic materials tend to be non-molecular...whereas covalent bonding can support discrete molecules..

