What are weak chemical bonds that form between polar molecules called?
1 Answer
May 22, 2016
dipole-dipole interaction
Explanation:
polar molecules are characterized by their net dipole moment resulting from the pull of electron density by an electronegative element from a less electronegative one.
For example, in a carbonyl group
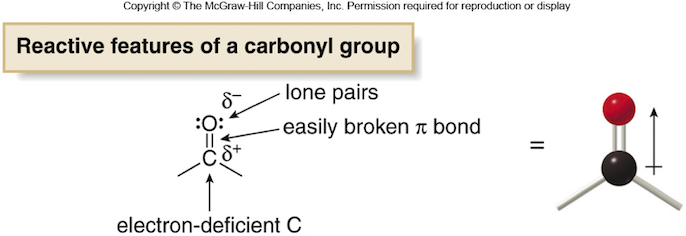
the attraction between the
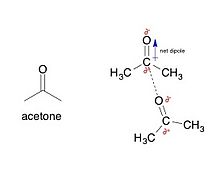
Image source: https://upload.wikimedia.org

