What evidence indicates that a base dissociates in water?
1 Answer
Apr 11, 2018
We know a base dissociates in water if the solution is able to conduct electricity.
Explanation:
When bases dissociate in water, two ions are formed.
For example, when
These ions are both charged and able to move in solution. So, because it is a charge carrier, it will be able to conduct electricity.
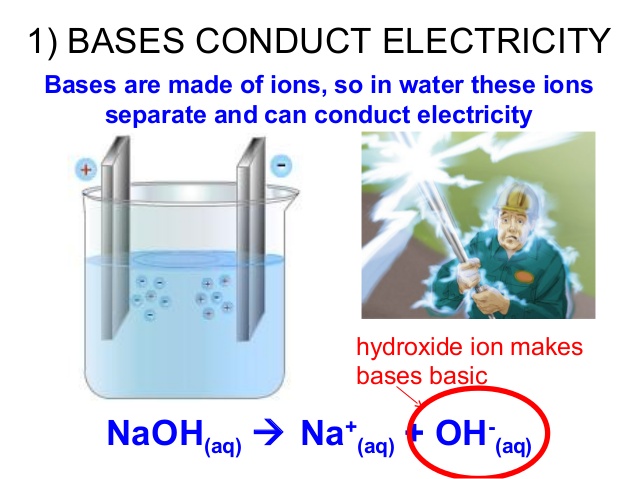
This means that we can test whether a base dissociates in water or not by running an electric current through it.

