What happens when alkenes are oxidized?
1 Answer
Alkenes are oxidised to give carbonyl compounds or carboxylic acids depending upon the condition.
Explanation:
So ozonolysis is an example of oxidative cleavage reaction that leads to the breaking
- Oxidative ozonolysis
- Reductive ozonolysis
Let me begin with oxidative ozonolysis. In this reaction,
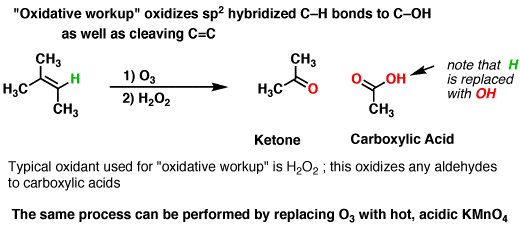
Now taking the second condition, i.e reductive ozonolysis.
In this case, oxygen is oxidized to intermediate form i.e carbonyl compound. The reductive workup is done with
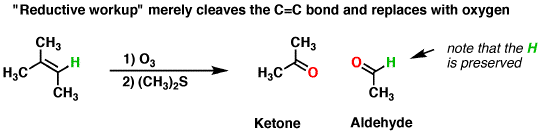
Hope it helps!!


