What is the mechanism for ozonoide formation?
1 Answer
Mar 4, 2018
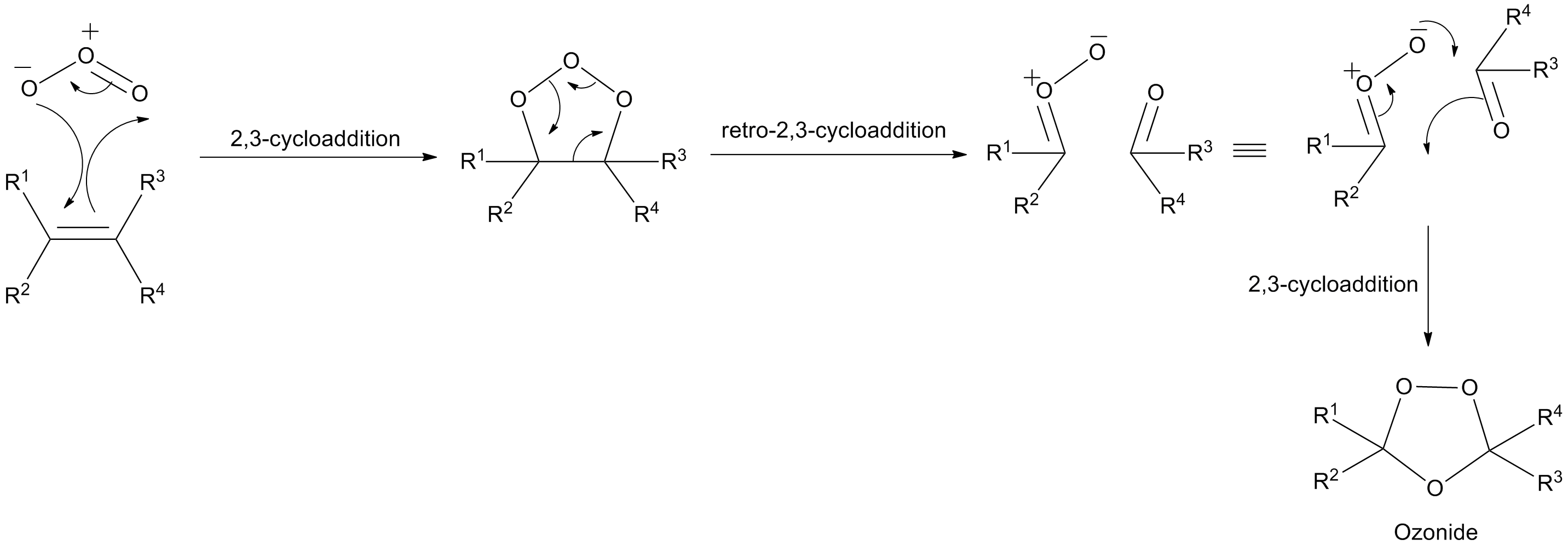
Explanation:
This involves 2,3-cycloaddition followed by retro-2,3-cycloaddition and then again 2,3-cycloaddition.

This involves 2,3-cycloaddition followed by retro-2,3-cycloaddition and then again 2,3-cycloaddition.

