What is a more substituted carbon from a less substituted carbon? What does substituted mean?
1 Answer
May 16, 2016
It just means that there are a different number of non-hydrogen groups on a particular atom. Below, (1) is a tertiary (
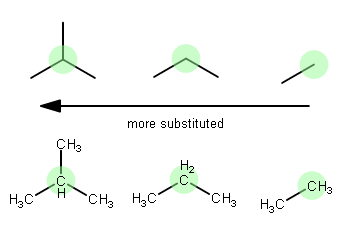
The highlighted carbon is currently being considered the "central" carbon.
Simply put...
- Since isobutane (1) has one more atom or group attached to the central carbon than propane (2), it is more substituted.
- Since propane (2) has one more atom or group attached to the central carbon than ethane (3), it is more substituted.

