What is an acid indicator?
1 Answer
An acid-base indicator is a large organic molecule, which is a WEAK acid.....
Explanation:
And the indicator is chosen so that its acid form has a distinct and characteristic colour with respect to its conjugate base.
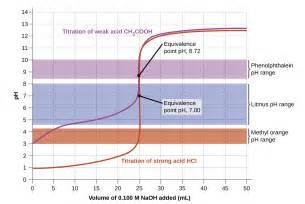
For the tritration of strong acid by a strong base, NEAR the ENDPOINT, the addition of 1 drop of titrant, approx.
The video below shows an experiment using an indicator derived from boiling red cabbage. A pigment from the cabbage called anthocyanin is what causes all of the different colors you see.
Other common indicators include:
bromothymol blue
thymol blue
methyl orange
bromocresol green
methyl red
phenol red
Hope this helps!


