What is an aromatic compound?
1 Answer
Jul 17, 2016
An aromatic compound is a COMPOUND that is 1) cyclic, 2) planar, and 3) has a conjugated π system containing
Explanation:
How to know whether a compound is aromatic or not?

Every π bond is counted as 2 electrons.
Here there is a planar ring with 3 π bonds, and each has two electrons.
So we have
A negative charge is counted as 2 electrons.
A positive charge is to be counted as 0 electrons.
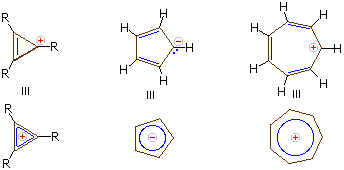
The 3-membered cation is aromatic because it has
The 5-membered anion is aromatic because it has
The 7-membered cation is aromatic because it has


