What is stoichiometry?
1 Answer
A very basic intro to stoich...
video from: Noel Pauller
Stoichiometry is the calculation of relative quantities of reactants and products in chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products.
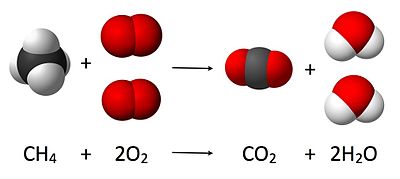
C
Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction.
as per the equation 1 mole of Methane , reacts with 2 moles of Oxygen to produce 1 mole of Carbon dioxide and 2 moles of Water.
if we double the amount of Methane and Oxygen to 2 moles and 4 moles respectively , the amount of carbon dioxide ad water we will get also get doubled up.
How many moles of chlorine gas (
(b) Using the equation (after it is balanced) above, determine the amount of product that can be produced from 24.7 g Na.
As per the equation 2 moles of sodium produces one mole of
If we start with 5 moles of Na , we will need 5 / 2 moles or 2.5 moles of
(b) As per the equation 2 moles of sodium ( mass 46 g) produces two moles of NaCl (116.8g)
we can say 46 g of Na on reaction with sufficient amount of Chlorine produces 116.8 g of NaCl.
1 g of Na on reaction produces (116.8 / 46 ) g of NaCl
24.7 g of Na on reaction will produce ( 116.8 / 46 ) x 24.7 g of NaCl
62 g of NaCl.


