What is the basic difference between Valence bond theory and Molecular orbital theory??? Very basics. Thank you.
1 Answer
DIFFERENCES
Valence bond theory assumes that electrons in a molecule are simply the electrons in the original atomic orbitals, with some used while bonding.
In other words, it does not account for the true distribution of electrons within molecules as molecules, but instead, treats electrons as if they are "localized" on the atoms themselves.
By drawing a Lewis structure and describing the electron distribution on that, you are using valence bond theory.
On the other hand, molecular orbital theory accounts for the "delocalization" of electrons (just like in
Instead, it says that the atomic orbitals combine to form the same number of new molecular orbitals as there were atomic orbitals (conservation of orbitals), and the electron distribution is based on that.
By working out a full molecular orbital diagram to see how the electrons are distributed then, you are using molecular orbital theory.
DISCOVERIES/CONCLUSIONS
As a result, a major discovery based on molecular orbital theory is that it correctly explains that
From the MO diagram of
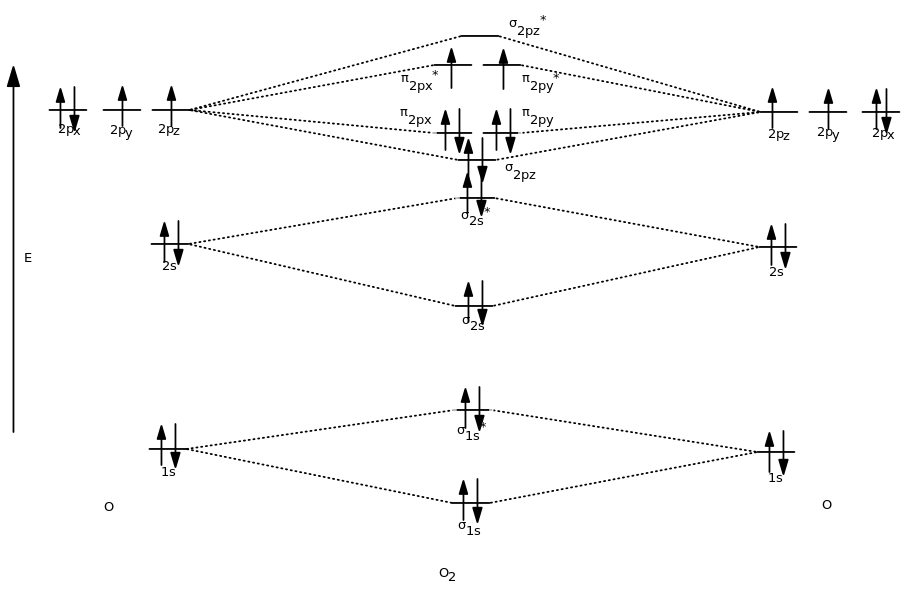
But if you simply look at the Lewis structure, like valence bond theory has you do, it's not obvious that there are any unpaired valence electrons at all.

