What is the difference between an ionic bond and a covalent bond?
1 Answer
From first principles, we regard the
Explanation:
.....And the
Metals are electron rich materials; they are good reducing agents, and typically form
On the other hand, non-metals are electron poor materials; they are good oxidizing agents, and typically form
I think you can see where this is going, because for every reduction, electron gain, there is an equal and corresponding electron loss, and thus metals can be typically oxidized to form discrete cations.....
The resultant salt, here
So we have dealt in part with ionic bonding. What about the
The modern covalent bond is conceived to be a region of high electron density between 2 positively charged atomic nuclei, such that the nuclei are attracted to the electron cloud electrostatically.
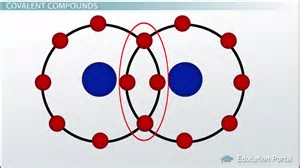
If we mapped electron density, (which your text will do), there is a region of high electron density BETWEEN the nuclei, which effects the covalent bond. Because, the bond is between just 2 atoms, covalent bonding generally results in discrete particles, i.e.
Anyway, none of this is ANY substitute for reading the chapter in your text. These are fairly simple ideas, and they reasonably easy to describe.

