What is the difference between nucleophilic substitution and electrophilic addition?
1 Answer
It's really in the terms "substitution" and "addition" that we find a meaningful difference.
NUCLEOPHILIC SUBSTITUTION
Nucleophilic substitution is when a nucleophile attacks an electrophilic site (i.e. a particularly electropositive site) and displaces a substituent in order to form a new molecule.
One example of such a reaction looks something like this (without consideration of
We can see that
We can conclude that nucleophilic substitution reactions will overall have had a leaving group leave from the substrate, due to the participation of a nucleophile.
ELECTROPHILIC ADDITION
Electrophilic addition is addition onto an electrophile without displacement.
One common electrophilic addition reaction is actually the halogenation of alkenes to generate vicinal dihalides. It's the dihalogen that acts as the electrophile.
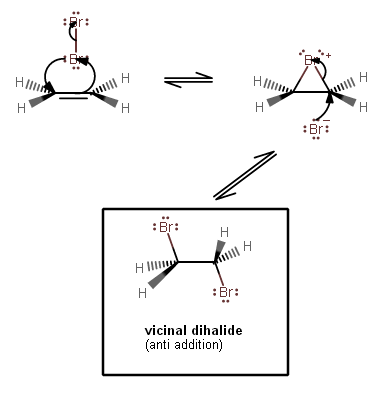
Initially the
That causes a London-Dispersion distortion in the electron cloud of
The bromonium intermediate is unstable, allowing
For this, we can say that electrophilic addition reactions will overall add something onto a previously electron-rich molecule, but the net result does not involve something having left the original substrate by the time the reaction is over.

