What is the dipole moment for azulene?
1 Answer
Oct 21, 2015
The dipole moment of azulene is
Explanation:
In the various resonance structures for azulene, the most stable contributors put negative charge in the 5-membered ring and positive charge in the 7-membered ring.
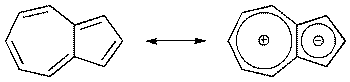 Azulene resonance
Azulene resonance
(from commons.wikimedia.org)
In effect, this gives an aromatic 6 π-electron five-membered ring fused to an aromatic 6 π-electron seven-membered ring, as we see in the above diagram.
This separation of positive and negative charge gives the molecule a dipole moment of

