Either your teacher is incorrect or your molecular formula is incorrect.
IF YOUR FORMULA IS CORRECT
#"C"_2"H"_6# has an #sp^3# hybridization on each carbon because of the four electron groups surrounding each carbon. It made four identical bonds in a perfect tetrahedral geometry, which means it needed four identical orbitals to make those bonds.
Each carbon has to hybridize one #2s# and three #2p# orbitals in order to generate four identical #sp^3# orbitals that are compatible in symmetry with hydrogen's #1s# orbitals.
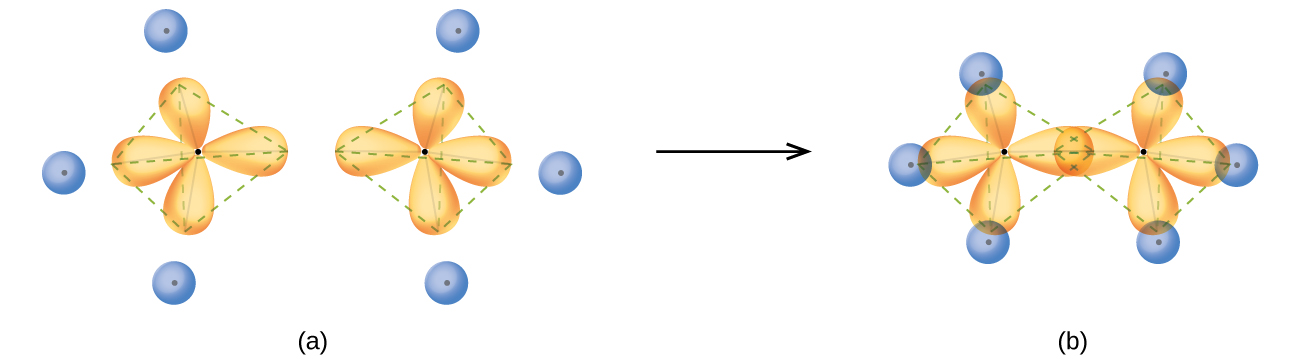
Therefore, each #"C"-"H"# bond in #"C"_2"H"_6# is between an #sp^3# of carbon (YELLOW) and a #1s# of hydrogen (BLUE sphere), i.e. an #sp^3-s# connection (the YELLOW/BLUE overlap in (b)), and each #"C"-"C"# bond is an #sp^3-sp^3# connection (the YELLOW overlap in (b)).
IF YOUR TEACHER WAS CORRECT ON THE HYBRIDIZATIONS
On the other hand, #"C"_2"H"_2# would match your teacher's observations.
With two electron groups on a POLYatomic molecule, each carbon requires only two #sp# lobes and hence only one #sp# hybridized orbital to bond with the other carbon AND a single hydrogen.
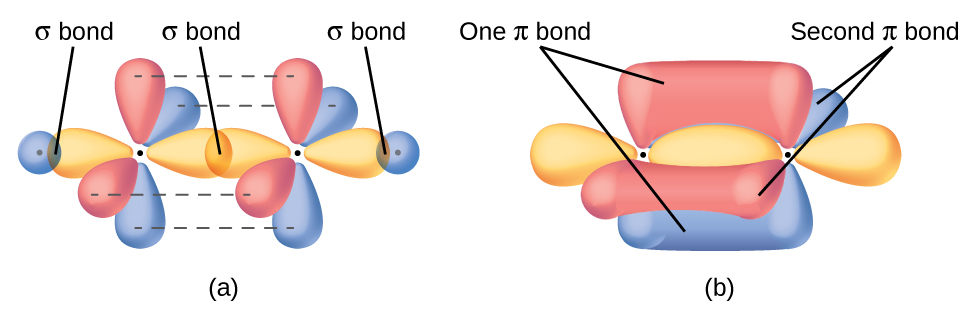
In this case we can see that:
- The #sp# of carbon 1 (YELLOW dumbbell) overlaps with one hydrogen's #1s# (BLUE sphere) to make a #\mathbf(sigma)# bond.
- The #sp# of carbon 1 (YELLOW dumbbell) overlaps with the #sp# of carbon 2 (YELLOW dumbbell) and vice versa to make a #\mathbf(sigma)# bond.
- The #sp# of carbon 2 (YELLOW dumbbell) overlaps with the second hydrogen's #1s# (BLUE sphere) to make a #\mathbf(sigma)# bond.
Each triple bond incorporates an additional #p_x"/"p_x# and #p_y"/"p_y# overlap between carbons 1 and 2, accounting for two #pi# bonds (i.e. #p-p# connections).
Hence, when including those two #p-p# #pi# bonds with the #sp-sp# #sigma# bond between carbons 1 and 2, we have accounted for the the triple bond between carbons 1 and 2.
(One triple bond = 1 #sigma# + 2 #pi# bonds)



