What is the hybridization of each carbon in this molecule?

1 Answer
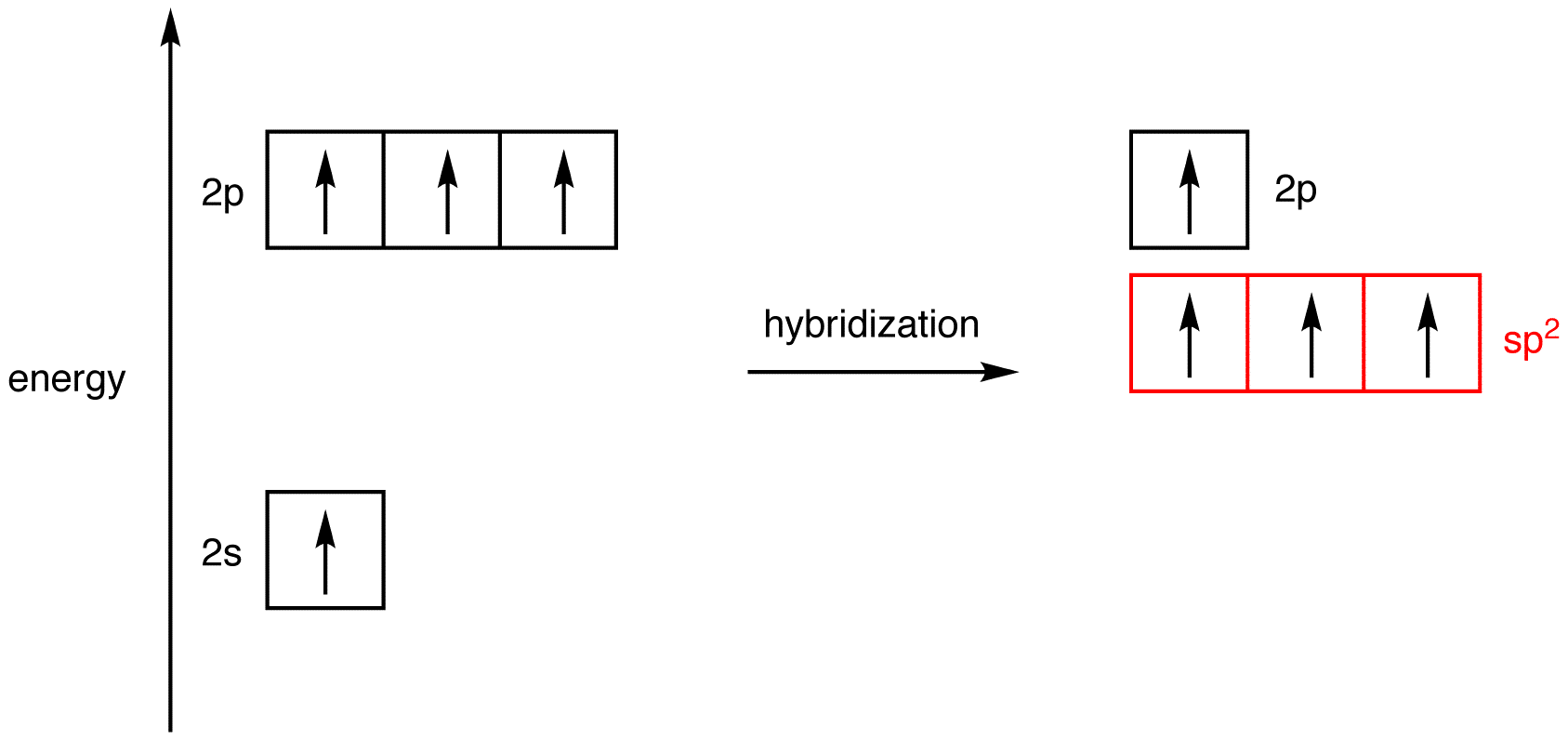
It is
Explanation:
This is because the first carbon has formed four bonds. So as you can see from the picture one electron from 2s orbital moves to the empty 2pz orbital. The 2s and the three 2p orbitals hybridise together and each orbital will be completed by adding one more electron from sharing with N, H, H, and the other C.
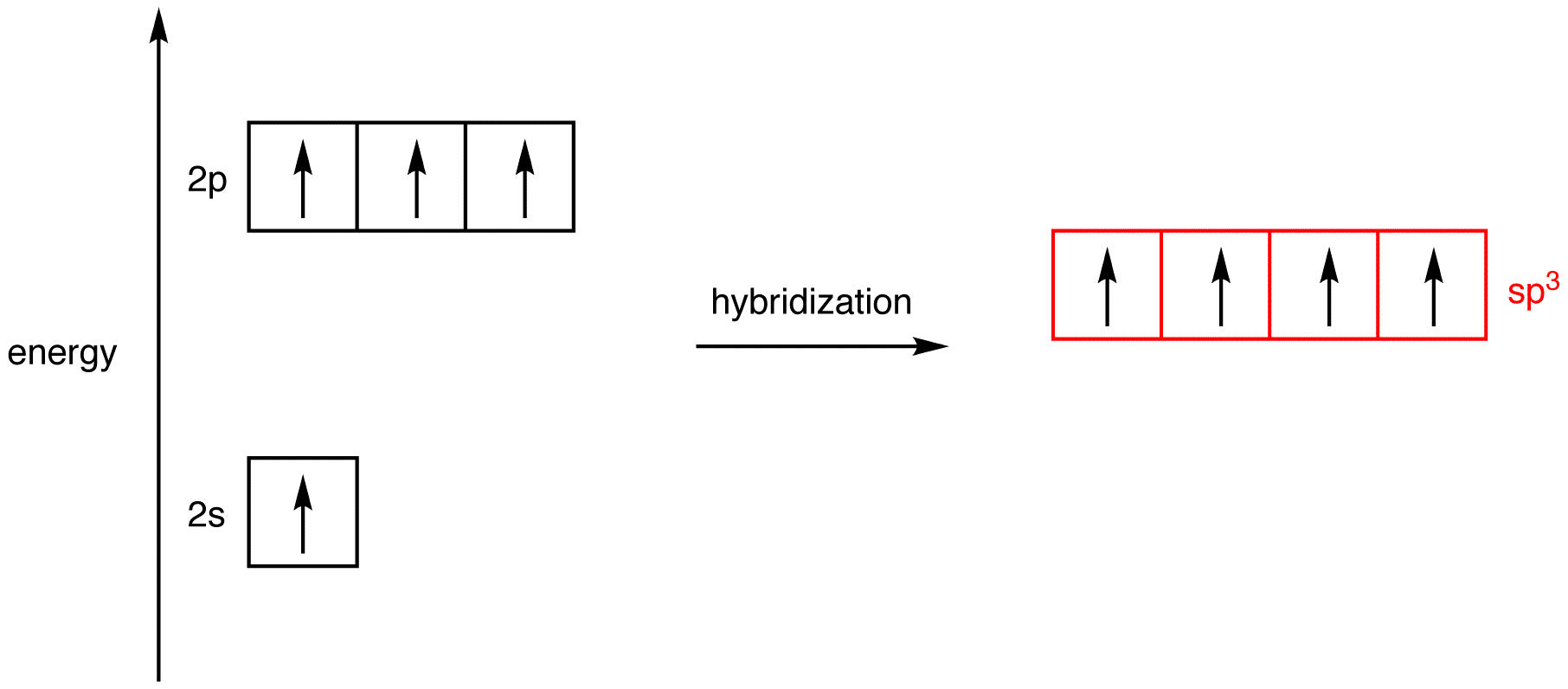
While the second carbon is
The unhybridised p orbital will be completed with the other sharing electron of the oxygen. This with form the