What is the Lewis electron dot formula (Lewis structure) of Nitrous Oxide (N_2O)?
1 Answer
I learned this by the counting-electron method, and then assigning formal charges to determine the most likely distribution of valence electrons.
The number of valence electrons available in the structure are:
(N :5 e^(-))xx 2 = 10 e^(-) O :6 e^(-)
10 + 6 = 16 total available valence electrons.
We have two nitrogens and one oxygen, which suggests that either we have oxygen in the middle or two nitrogens in a row.
Notice how if you had oxygen in the middle, the formal charges of both nitrogens have no way of being distributed well without exceeding 8 electrons for oxygen:
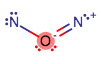
One way of determining formal charges is:
"Formal Charge" = "Expected Electrons" - "Owned Electrons"
Since the left nitrogen "owns" five valence electrons (two lone pairs and one from the
Since the right nitrogen "owns" four valence electrons (one lone pair gives two electrons; then, two electrons from the
Since the oxygen "owns" seven valence electrons (two lone pairs gives four electrons; then, one electron from the left
Oxygen has TEN, but it cannot have more than EIGHT valence electrons, so that structure is ruled out.
Thus, one of the nitrogens must be in the middle.
Now we can get two plausible possibilities, which are both linear molecular geometries (NOT bent!!! Two electron groups!):
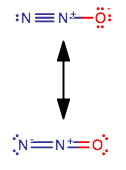
CHALLENGE: Can you determine why the formal charges are how they are? Based on electronegativity differences between oxygen (

