What is the Lewis structure for resonance form of ClO_2^-?
1 Answer
Dec 16, 2017
Well we has got................
Explanation:
And so....we gots
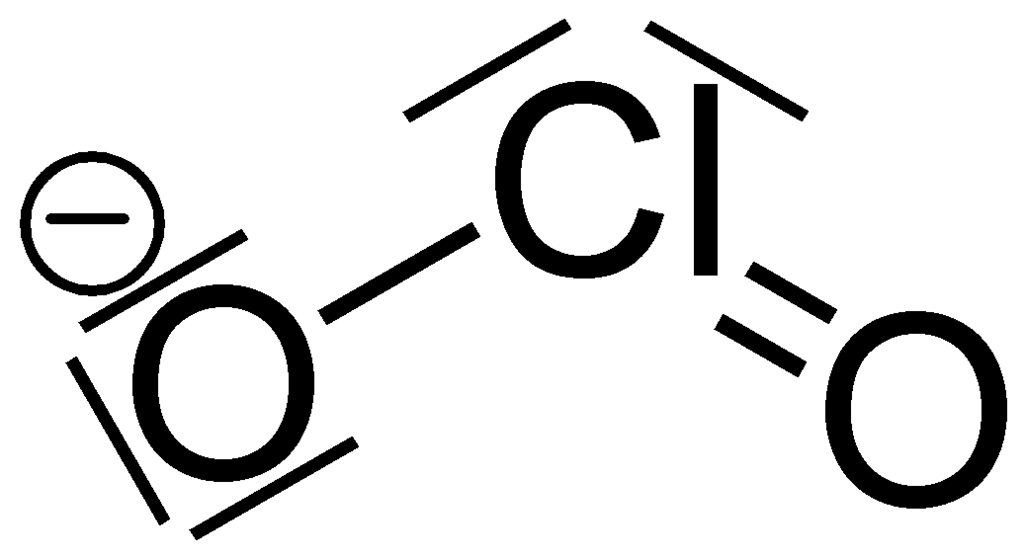 en.wikipedia.org
en.wikipedia.org
The electronic geometry around chlorine is tetrahedral. The molecular geometry is bent with

