What is the Lewis structure of CO?
1 Answer
This often looks wrong to a student who is used to seeing double bonds on oxygen.
Students are typically taught an electron-counting method, which goes as follows:
- Count the number of valence electrons per atom.
- Draw out a predicted atom connectivity.
- Place all electrons in predicted spots.
- Where there are electron pairs, construct one bond line for each electron pair. (There are two
#pi# bonds and one#sigma# bond in a triple bond, one#sigma# and one#pi# bond in a double bond, and one#sigma# bond in a single bond.) - Assign formal charges, and fix the resonance structure by moving electrons and bond lines around until the formal charges are minimized.
Formal charges can be defined simply by:
#"Charge = valence electrons - owned electrons"#
With
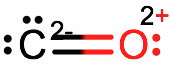
But that is not likely correct. Carbon is less electronegative than oxygen, so it won't be happy with having more electrons than oxygen. The tension of the unhappy oxygen really wanting the electrons destabilizes this particular resonance structure.
Another option is:

But carbon does not have an octet, so this is not realistic. Finally, we land upon the only other rational possibility:

Here, carbon is less unhappy; both atoms have octets, and the electrons are more evenly distributed, minimizing the formal charges AND minimizing the energy overall in this major resonance structure. So this is correct.

