What is the Lewis structure of N2?
1 Answer
Oct 30, 2015
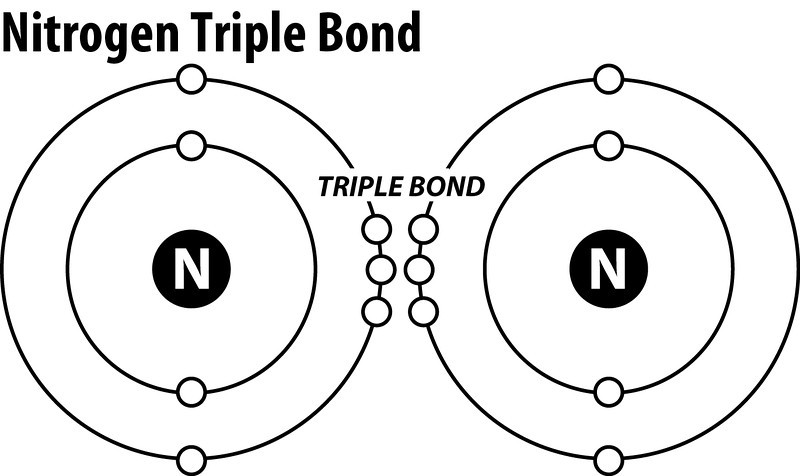
Explanation:
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since
Here is the electron dot structure for a single

The total number of valence electrons between the two


