What is the major product of the Stork enamine synthesis, that is, the reaction of cyclopentanone with pyrrolidine, heat, #CH_3CH_2Br#, #H^+#, and #H_2O#?
1 Answer
Apr 22, 2017
The major product is 2-ethylcyclopentanone.
Explanation:
The reaction is often called the Stork enamine synthesis.
Step 1. Formation of an enamine.
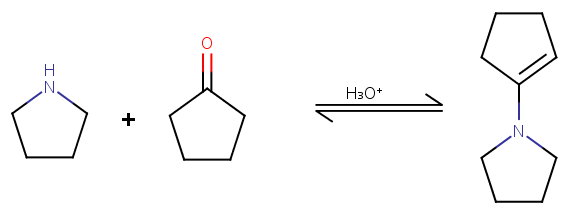
Step 2.
The double-bonded carbon in the enamine has a partial negative charge, so it can act as a nucleophile and displace the
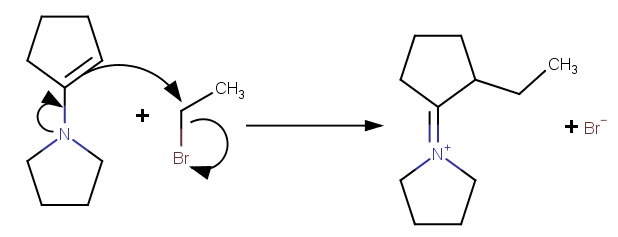
Step 3. Hydrolysis of the iminium salt
The iminium salt is hydrolyzed to regenerate the ketone and the amine.
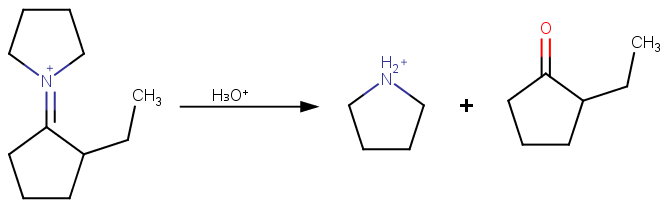
Note: The reaction is done this way because direct alkylation of the cyclopentanone enolate gives multiple alkylation, but the enamine stops at one alkylation.

