What is the mechanism for the hydrolysis of an anhydride into a carboxylic acid using HCl as a catalyst?
1 Answer
Feb 22, 2017
This is a fairly standard mechanism (or mechanistic pattern) that you should get to know.
It is practically identical for anhydrides as it is for acyl halides (particularly acyl chlorides) and esters, and similar variations are seen in the acid-catalyzed hydrolysis of nitriles, amides, etc, wherein the electron-dense atom (e.g.
It is perfectly acceptable to assume one starts with hydronium when a strong acid like
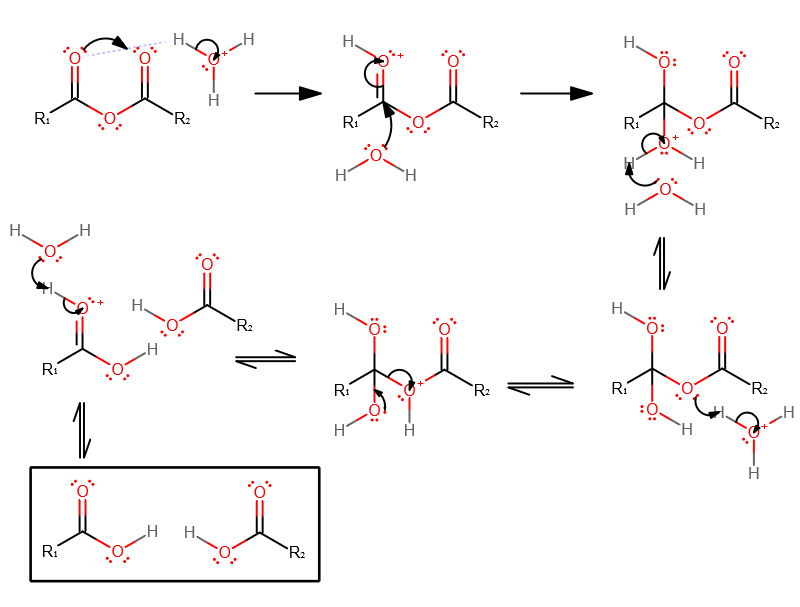
- The electron-dense carbonyl oxygen acquires a proton from the strong acid. Either carbonyl oxygen is fine, just not the ether oxygen.
- Water then nucleophilically attacks the partially positive carbon, as oxygen withdraws electron density to break the carbonyl bond.
- Proton transfer pt1.
- Proton transfer pt2.
- Tetrahedral collapse. You may choose which hydroxyl group does so, but it is usually the one that originated from the nucleophile that is typically used.
- Regenerate the catalyst.
NOTE: You must replace

