What is the resonance structure for benzyl alcohol (C6H5CH2OH) ?
1 Answer
Aug 11, 2018
Well, the hydroxyl group is NOT conceived to be delocalized around the aromatic ring.....
Explanation:
Why, because the benzyl is electron precise...
Of course, the phenyl group engages in the typical resonance stabilization...
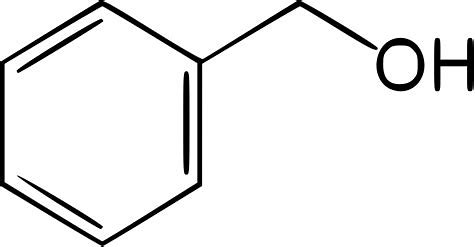
Induction activates ALL the carbon centres of the phenyl ring towards aromatic substitution. The ortho and para positions are ESPECIALLY activated..

