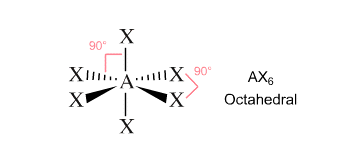
What is the smallest bond angle in #SF_6# ? A) 109.5° B) 60° C) 120° D) 90°
1 Answer
The answer is (D)
Explanation:
Sulfur hexafluoride,
VSEPR Theory states that these six regions of electron density, which is what the single bonds that exist between sulfur and the fluorine atoms are, will arrange themselves in 3-D space as far apart from each other as possible.
The configuration that maximizes the distance between these regions of electron density resembles an octahedron. Two fluorine atoms will occupy axial positions and four fluorine atoms will occupy ecuatorial positions.
Sulfur hexafluoride will have an octahedral molecular geometry.
The four ecuatorial bonds will be at a