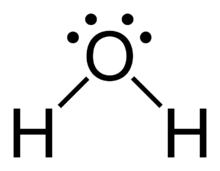
What is the steric number for the #"O"# atom in water?
1 Answer
Explanation:
An atom's steric number tells you how many regions of electron density surround the atom in a given molecule.
A region of electron density is simply
- a single, double, or triple bond
#-># all three count as one region of electron dnesity- a lone pair of electrons
This means that in order to find an atom's steric number, all you have to do is see how many lone pairs of electrons it has and to count the number of bonds it forms with other atoms.
A good starting point here will be to draw the Lewis structure of water,
Each water molecule contains
- one from each of the two hydrogen atoms
- six from oxygen
The oxygen atom will be the central atom. It will form two single bonds, one with each hydrogen atom, that account for
As you can see, the oxygen atom is surrounded by
As a result, the atom's steric number will be equal to