What major alkene product is produced by the dehydration of the following alcohols?
a. Cyclohexanol
b. 1-Methylcyclohexanol
c. 2-Methylcyclohexanol
d. 2,2-Dimethylcyclohexanol
e. 1,2-Cyclohexanediol
a. Cyclohexanol
b. 1-Methylcyclohexanol
c. 2-Methylcyclohexanol
d. 2,2-Dimethylcyclohexanol
e. 1,2-Cyclohexanediol
1 Answer
Here's what I get.
Explanation:
a) Cyclohexanol
Dehydration of an alcohol removes the
 classes.kvcc.edu
classes.kvcc.edu
b) 1-Methylcyclohexanol
Dehydration of an alcohol gives the more stable alkene (more highly substituted) as the major product.
 butane.chem.uiuc.edu
butane.chem.uiuc.edu
The major product is 1-methylcyclohexene and methylenecyclohexane is the minor product.
c) 2-Methylcyclohexanol
The more stable (major) alkene product is 1-methylcyclohexene and the minor product is 3-methylcyclohexene.
 academics.wellesley.edu
academics.wellesley.edu
d) 2,2-Dimethylcyclohexanol
The initially formed 2° carbocation undergoes a methyl shift to form a more stable 3° carbocation, which loses a proton to form 1,2-dimethylcyclohexane as the major product.
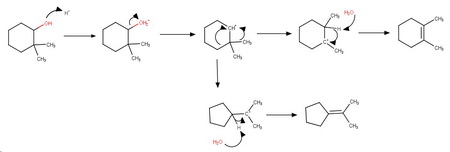 Mechanism
Mechanism
The 2° carbocation can rearrange by an alkyl shift to form another 3° cation that loses a proton to form isopropylidenecyclopentane as a minor product.
e) Cyclohexane-1,2-diol
The initial product is an enol, which quickly tautomerizes to the more stable ketone, cyclohexanone.
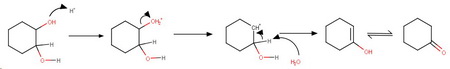 Mechanism
Mechanism

