What opens epoxides?
1 Answer
You could either do it in acid or in base.
EPOXIDES IN BASE
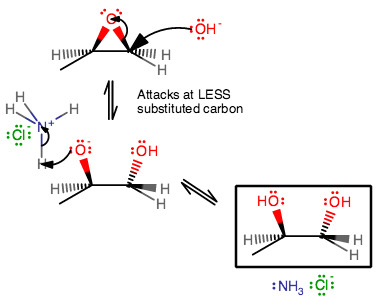
It is more intuitive in base, where something like
The choice of that carbon is fairly intuitive since that carbon is less sterically hindered and thus generally more susceptible to second-order kinetics.
At the same time, something less obvious is that the more-substituted
Afterwards, a weak acid like
EPOXIDES IN ACID
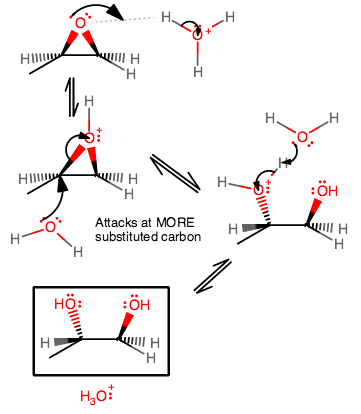
In acid, however, once the oxygen is protonated, there is a seemingly counterintuitive process going on: Why would the MORE substituted carbon be the target of the nucleophilic attack?
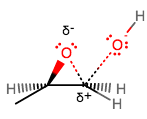
Although the oxygen bears the positive formal charge, it is one of the carbons directly connected to the oxygen that is more electropositive (remember carbon is less electronegative, and thus more electropositive), but which one?
The more substituted carbon is more affected by hyperconjugation from a
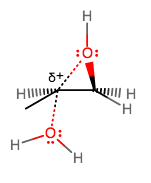
That implies a longer
So, despite there being more steric hindrance at the more substituted carbon, a weak nucleophile like water is justified in attacking there to open the ring because the electrophilic carbon is more stabilized in the transition state at the more substituted carbon.

