What type of bonding around a central atom would result in a trigonal planar molecule?
1 Answer
Jan 16, 2017
3 atoms around a central atom with no lone pairs. Two single bonds, and one double bond.
Explanation:
A trigonal planar molecule requires that there are no lone pairs disrupting the molecule's symmetry. This ensures that all three atoms lie on the same plane. An example of this configuration is the Carbonate ion (
In order to abide by the octet rule, this means that there will be two single bonds and one double bond on the central atom. Here's the Lewis structure drawing of a carbonate ion as an example.
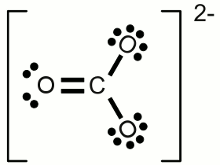
Here's what the Carbonate molecule would look like as an actual molecule: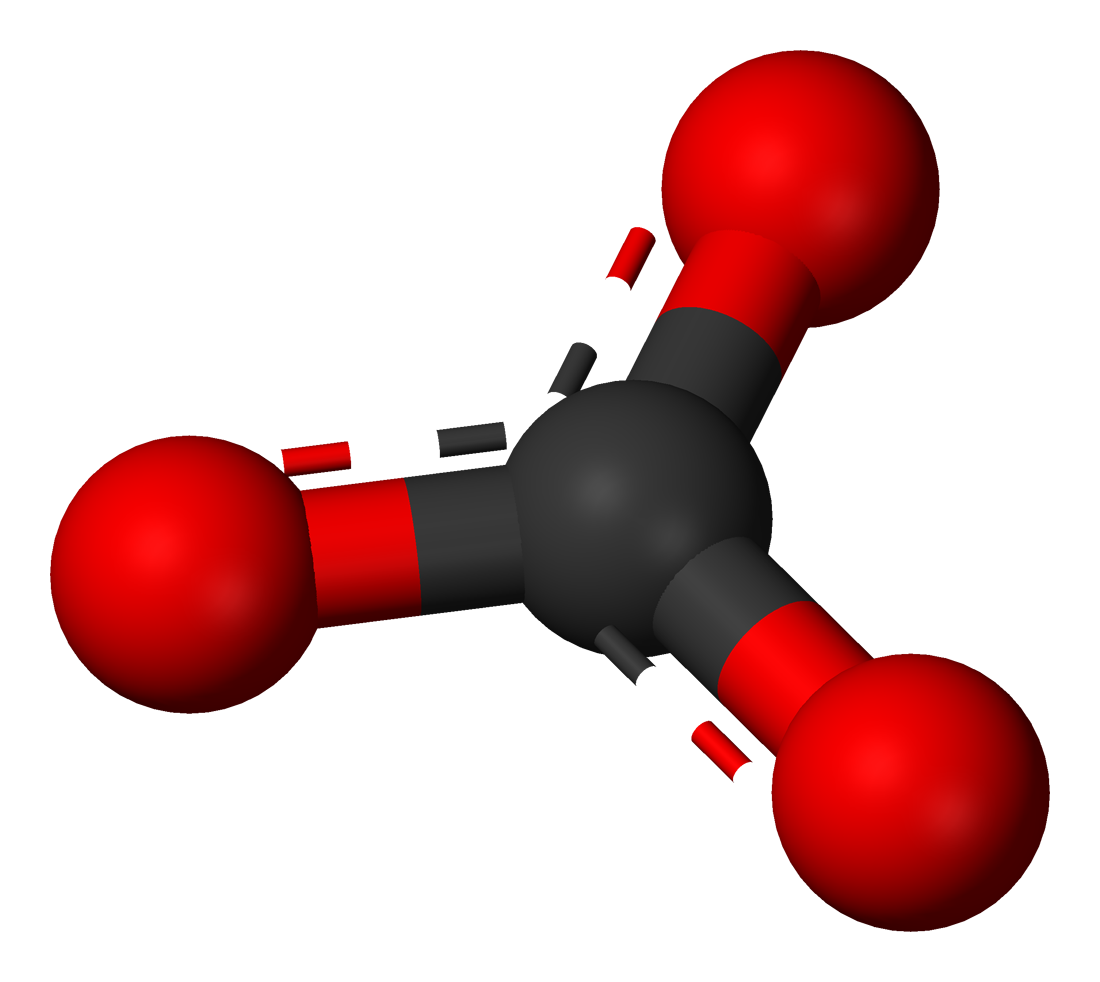
Notice how the three oxygen atoms are all on the same plane, showing the trigonal planar geometry of the molecule.
