What word or two-word phrase describes the shape of the formaldehyde (#CH_2O#) molecule?
1 Answer
Dec 5, 2016
From counting valence electrons:
- Carbon has four valence electrons, so it tends to make four bonds in covalent compounds.
- Oxygen has six valence electrons, but as it tends to accept two, it tends to make two bonds in covalent compounds.
- Hydrogen is almost always going to make only one bond, given that it only has one valence electron.
Eventually, you should get that formaldehyde looks like this:
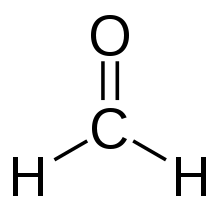
You can add the two remaining valence electrons onto
Since the
You can draw a triangular plane onto this molecule, and there are three electron groups (which are all bonding groups), so we call this molecular geometry around carbon trigonal planar.

