When 1-iodo-1-methylcyclohexane is treated with NaOCH_2CH_3 as the base, the more highly substituted alkene product predominates. When KOC(CH_3)_3 is used as the base, the less highly substituted alkene predominates. Why? What are the structures of the two products?
1 Answer
Mar 3, 2015
The less highly substituted alkene predominates with potassium t-butoxide because the t-butoxide ion is sterically hindered.
Explanation:
1-iodo-1-methylcyclohexane is a tertiary alkyl halide.

It tends to undergo E1 eliminations to give the most stable product — the most highly substituted alkene — 1-methylcyclohexene.
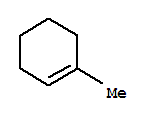
The t-butoxide ion is a strong base, but it is bulky. It is highly sensitive to steric interactions.
In an E2 mechanism, it attacks the least hindered, most accessible α-hydrogen it can find — an
The product is methylenecyclohexane.


