Which diastereomer of 1-bromo-4-t-butylcyclohexane, the cis or the trans, undergoes elimination more rapidly when treated with sodium ethoxide?
1 Answer
The cis-isomer undergoes elimination more readily.
Explanation:
The cis-isomer undergoes elimination more readily.
Since the base is sodium ethoxide, the mechanism is E2.
In an E2 elimination, the leaving group and the β hydrogen must go through an antiperiplanar transition state.

Now let's look at the structures of cis- and trans-1-bromo-4-t-butylcyclohexane.
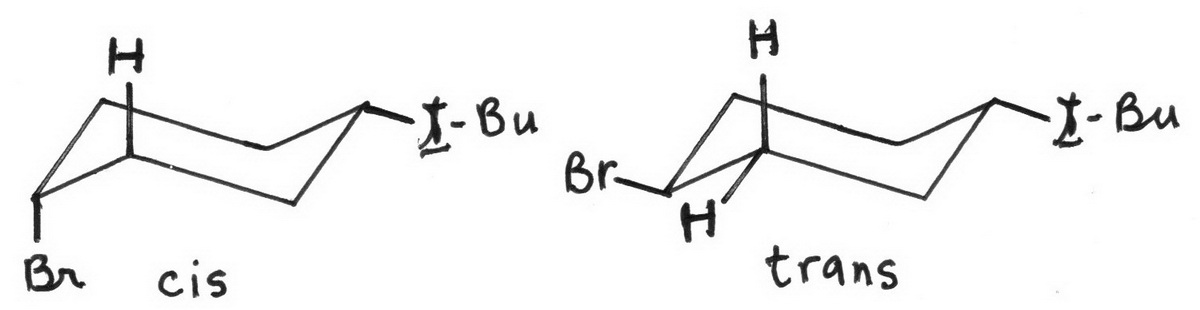
To avoid 1,3-diaxial interactions, the bulky t-butyl group must be in an equatorial position.
Only the cis isomer has the Br and the β hydrogen in an antiperiplanar (trans diaxial) arrangement.
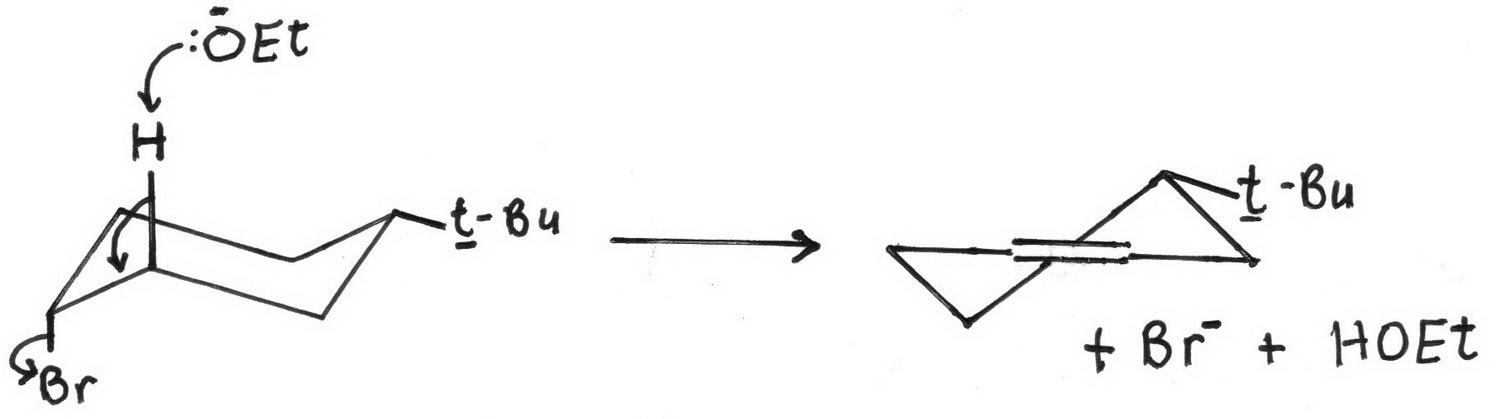
So the trans isomer will undergo more rapid elimination.

