Which of the arrangements of Bond Order is correct for the following?
NO_2^+>NO_2^(-)>NO_3^(-) NO_3^-)>NO_2^+>NO_2^- NO_2^+=NO_2^-)>NO_3^- NO_2^-)>NO_2^+>NO_3^-
Please explain in details.. Thank you!
NO_2^+>NO_2^(-)>NO_3^(-) NO_3^-)>NO_2^+>NO_2^- NO_2^+=NO_2^-)>NO_3^- NO_2^-)>NO_2^+>NO_3^-
Please explain in details.. Thank you!
1 Answer
The correct arrangement of bond orders is
Explanation:
The bond order (
It takes two shared electrons to form a bond, so
The structure of the nitronium ion is
There are two
Each bond is on average a double bond.
The structure of the nitrite ion is
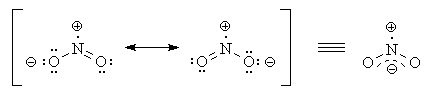 www.madsci.org
www.madsci.org
Each contributor to the resonance hybrid has an
Each bond is on average a "1½ bond".
The three contributors to the resonance structure of nitrate ion are
 www.mikeblaber.org
www.mikeblaber.org
Each contributor to the resonance hybrid has an
Each bond is on average a "1⅓ bond".
∴ The correct arrangement of bond orders is

