Which type of intermolecular forces exist between (a)H_2S molecules (b)Cl_2 and C Cl_4 molecules?
1 Answer
(a) Dipole-dipole forces
(b) London Dispersion Forces
Explanation:
(a)
Sulfur atoms have 6 valence electrons and hydrogen atoms have 2 valence electrons. So, the Lewis structure of
 http://wiki.chemeddl.org/resources/models360/models.php?pubchem=402
http://wiki.chemeddl.org/resources/models360/models.php?pubchem=402
We see that the central sulfur atoms has 4 entities around it. Two hydrogen atoms, and two lone non-bonding electron pairs. According to VSEPR theory, this means that the shape of the molecule is bent and because of the asymmetrical shape, the molecule is polar .
The intermolecular force which polar molecules take part in are dipole-dipole forces.
(b)
Chlorine atoms have 7 valence electrons. So, the Lewis structure of
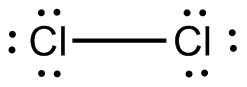 https://socratic.org/questions/how-are-covalent-bonds-represented-in-lewis-dot-diagrams
https://socratic.org/questions/how-are-covalent-bonds-represented-in-lewis-dot-diagrams
Here, there is no central atom, and both atoms are of the same element. This means that the molecular shape is linear, and because of the symmetrical shape, the molecule is nonpolar.
Nonpolar molecules cannot take part in dipole-dipole interactions due to the lack of permanent dipoles, and there are no other characteristics that give this molecule the ability to have stronger intermolecular forces. This means that
Carbon atoms have 4 valence electrons, so the Lewis structure for
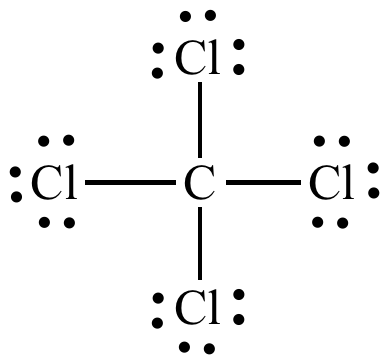 http://www.chem.ucla.edu/~harding/IGOC/C/carbon_tetrachloride.html
http://www.chem.ucla.edu/~harding/IGOC/C/carbon_tetrachloride.html
We can see here that the central carbon atom has four atoms around it, and no non-bonding lone pairs. According to VSEPR theory, this means that the molecule is tetrahedral in shape, and therefore symmetrical. This means that the molecule is overall nonpolar .
Nonpolar molecules cannot take part in dipole-dipole interactions, and there are no other characteristics that give this molecule the ability to have stronger intermolecular forces. This means that
Because both

