Why are dipole dipole forces attractive?
1 Answer
Jan 30, 2017
Electric dipole interactions are attractive because dipoles can orient themselves in a way that brings opposite charges closer that like charges.
Explanation:
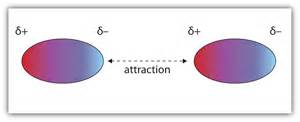
Picture one polar molecule in a compound or solution (such as water). Since a polar molecule will have a positive end and a negative end, the positive end of one molecule can attract the negative end of a nearby molecule (and spin that molecule around, if necessary) so that the negative end of the second molecule is brought closer than the positive end of the first.
This causes a net force between the two molecules that is attractive.
Check the image below: