Why are some epoxides toxic?
1 Answer
Jun 10, 2016
Most epoxides are toxic because their high reactivity makes them mutagenic.
Explanation:
The 3-membered epoxide ring is highly strained, so it is susceptible to ring opening by nucleophiles.
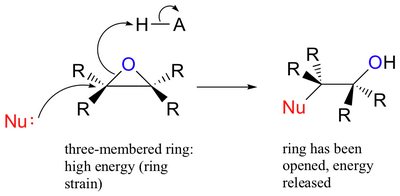 Epoxide opening
Epoxide opening
(from chemwiki.ucdavis.edu)
Common nucleophiles are
In fact, reactions with
There is little evidence that epoxides cause cancer. However, most epoxides are mutagenic.
They form covalent bonds to guanine, and the adduct prevents proper G-C base pairing.
The genetic code is misread, and the resulting changes can be passed on to future generations.

