Why do ionic radius increase?
1 Answer
Jul 25, 2018
In what context...?
Explanation:
Anionic radius should certainly be larger than the radius of the parent atom. Cationic radius should certainly be SMALLER than that of the parent atom. Why? Because, reasonably, both atomic and ionic radii are DEFINED by the radius of the valence electron, And of course a redox process adds or removes an electron from an atom, and thus the ionic radius will change markedly with respect to the radius of the parent atom.
And so as scientists we should look at the data....
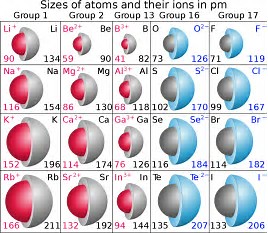
The answer is in
Do the given data support what we have argued?

