Why does H bind to the first Carbon, i know that H binds to the C that already has more H's attached to it. But i still dont get it because the first Carbon has only two hydrogens?
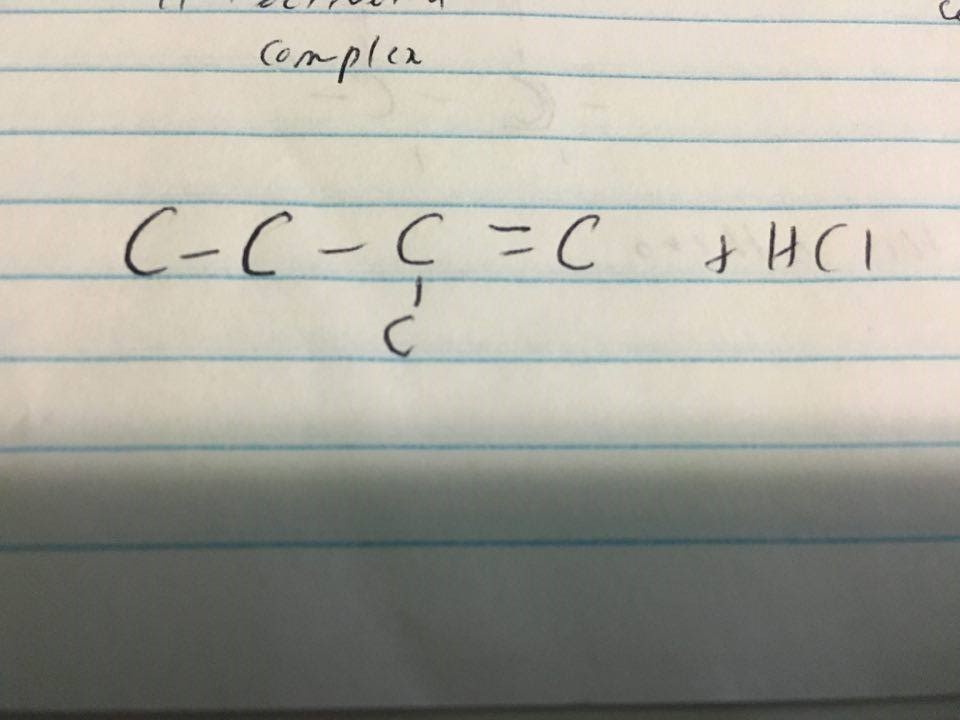
1 Answer
Well, to see why
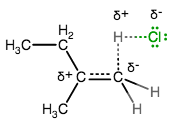
As the alkene donates its
The more stable carbon can more easily hold a positive charge, and the OTHER carbon thus gets protonated.
Both carbons are
CARBOCATIONS PRESENT
A primary (
By definition, carbon-1 would have become a primary carbocation intermediate---just stare at it for a while and you should see that the
#"R"# group is everything to the left of carbon-1.
A tertiary (
In this case, looking at carbon-2, your
#"R"# groups are#"H"_3"C"# ,#"H"_3"C"-"H"_2"C"# , and#"CH"_2# . Therefore, carbon-2 is by definition going to become a tertiary carbocation intermediate.
CARBOCATION STABILITY
Okay, so which is more stable? If you recall, your professor should have talked about hyperconjugation, where the surrounding
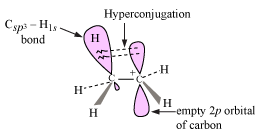
This interaction distributes electron density from the
(It's like distributing an even amount of water in multiple glasses instead of pouring it all into one glass.)
Since there are three
In fact, we can make this statement about the carbocation stabilities:
#3^@ > 2^@ > 1^@ > "methyl"#
CONCLUSIONS
So, carbon-2, being tertiary, is more favorably positively charged, and thus, the proton must go on carbon-1 to prevent it from forming a less-stable intermediate. The carbon that the proton does not go onto becomes positively charged!
This correlates with your idea that the proton normally goes onto the carbon with more hydrogens---carbon-2 has no hydrogens directly attached to it at all. Carbon-1 has two.
So even by that logic (which, though simpler, is also correct), carbon-1 should indeed be protonated via Markovnikov addition.

