Why does orbital hybridization take place?
1 Answer
See explanation.
Explanation:
I will try to explain hybridization from its simplest concept.
Keep in mind that hybridization occurs to satisfy the molecular geometry in order to achieve the lowest state of energy of the molecule.
Let us consider the simplest example which is methane:
Someone, might imagine that for four hydrogen atoms to be around the carbon atom the Lewis structure would be this:
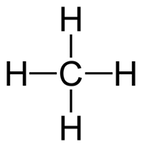
The way that this molecule is drawn, the bond angles are
However, the experimental results have shown that the molecular geometry around a carbon atom is tetrahedral and that the single bonds angles are
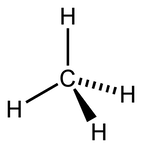
which cannot happen if the carbon atom uses its
Therefore, we can use the following:
Tetrahedral:
Trigonal planar:
Linear:

