Why is aldehyde hydrogen not acidic?
1 Answer
Jul 28, 2016
Because it would make the molecule far too unstable. It probably does have its own
If we took acetaldehyde as an example:
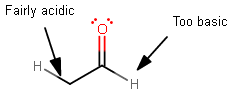
The
If you attempt to deprotonate acetaldehyde, clearly the second option is better.
- It doesn't exceed an octet for oxygen.
- The electrons are able to reasonably delocalize. The electronegativity of oxygen is greater than that of carbon, so oxygen is capably negatively charged.
- The equilibrium on the first one is skewed far to the left, whereas that on the second one is skewed enough to the right.
In fact, it is these

