Why is the maximum density of water at 4°C?
1 Answer
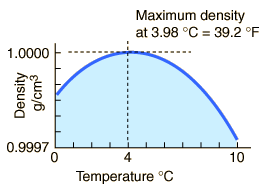
The maximum density of water occurs at 4 °C because, at this temperature two opposing effects are in balance.
Explanation:
In ice, the water molecules are in a crystal lattice that has a lot of empty space.
When the ice melts to liquid water, the structure collapses and the density of the liquid increases.
At temperatures well above freezing, the molecules move faster and get further apart. The density decreases as temperature increases.
At temperatures near 0 °C, the water still contains many ice-like clusters. These clusters are free to move relative to each other, so water is still liquid.
The clusters still have empty spaces, so they decrease the density of the liquid.
The molecules of the water are closer together, and this increases the density of the liquid.
Now, let's cool the water.
As the temperature of warm water decreases, the water molecules slow down and the density increases.
At 4 °C, the clusters start forming.
The molecules are still slowing down and coming closer together, but the formation of clusters makes the molecules be further apart.
Cluster formation is the bigger effect, so the density starts to decrease.
Thus, the density of water is a maximum at 4 °C.